


 All payments are encrypted via SSL
All payments are encrypted via SSL
 Full Refund if you haven't received your order
Full Refund if you haven't received your order
Coated Tablets from white to pale cream color, round, biconvex, with the words "Janssen" on one side and "M / 10" on the other; on a break - white.
1 pill contains Domperidone 10 mg;
Excipients: lactose, corn starch, microcrystalline cellulose, pregelatinized potato starch, polyvidone, Magnesium stearate, vegetable oil, hydrogenated, sodium lauryl sulfate, hypromellose;
10 or 30 pieces in blister, 1 blister in a carton box.
Lingual tablets white or almost white, round, instant.
1 pill contains domperidone 10 mg;
Excipients: gelatin, mannitol, aspartame, mint flavor.
10 pieces. in blisters, 1 or 3 blisters in a carton box.
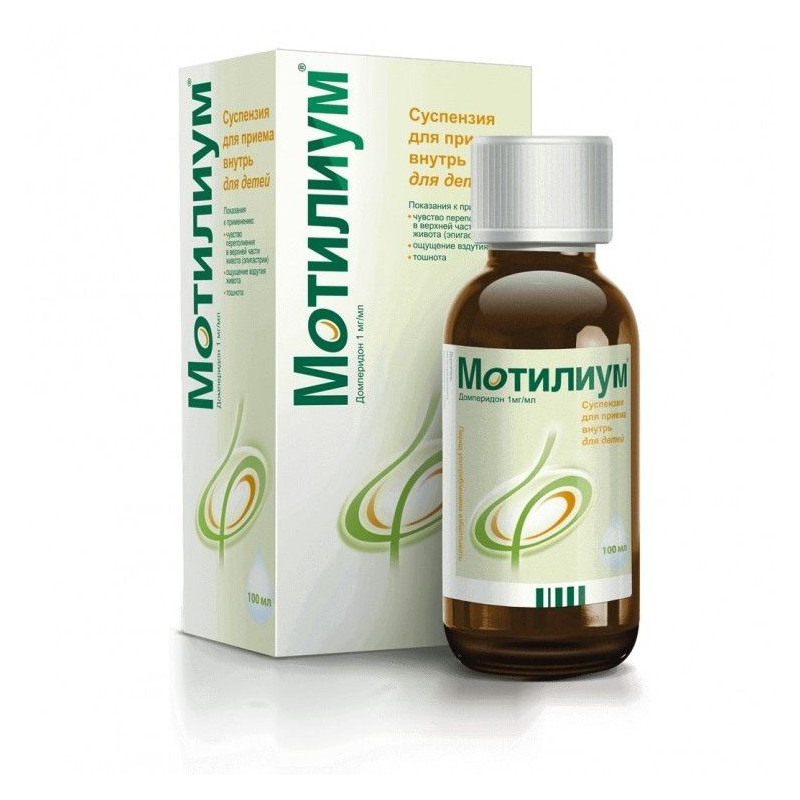
Oral suspension uniform, white color.
5 ml of the suspension contain domperidone 5 mg;
Excipients: sodium saccharinate, microcrystalline cellulose, sodium carboxymethylcellulose, sorbitol, methyl parahydroxybenzoate, propyl parahydroxybenzoate, sodium hydroxide, polysorbate, purified water.
100 or 200 ml in bottles, 1 bottle complete with a graduated pipette for 5 ml or a measuring cap for 10 ml in a cardboard box.
suspenfor oral administration - P №014062 / 01-2002, 03.06.02
tabl. by. - №014853 / 01-2003, 03/25/2003
Antiemetic drug, GI motility stimulator. Domperidone is a dopamine antagonist that, like Metoclopramide and some antipsychotics, has antiemetic properties. However, unlike these drugs domperidone poorly penetrates through the BBB. Domperidone use is rarely accompanied by extrapyramidal side effects, especially in adults, but domperidone stimulates the secretion of prolactin from the pituitary gland. The antiemetic effect is probably due to a combination of peripheral (gastrokinetic) action and antagonism of dopamine receptors in the trigger zone of chemoreceptors.
When administered domperidone increases the duration of antral and duodenal contractions, accelerates gastric emptying - the release of liquid and semi-solid fractions in healthy people and solid fractions in patients, when this process was slowed down, and increases the pressure of the sphincter of the lower esophagus in healthy people.
Domperidone has no effect on gastric secretion.
- complex of dyspeptic symptoms, often associated with delayed gastric emptying, gastroesophageal reflux, esophagitis (feeling of fullness in the epigastrium, feeling of bloating, pain in the upper abdomen, belching, flatulence, nausea, vomiting, heartburn and regurgitation);
- nausea and vomiting of functional, organic, infectious origin, caused by radiotherapy, drug therapy or diet disorder;
- nausea and vomiting caused by dopamine agonists when used in Parkinson’s disease (such as L-dopa and bromocriptine);
- regurgitation syndrome, cyclic vomiting, gastroesophageal reflux and other disorders of gastric motility in children.
- Gastrointestinal bleeding;
- mechanical obstruction or perforation, in which the stimulation of the motor function of the stomach can be dangerous;
- prolactin-secreting pituitary tumor (prolactinoma).
- simultaneous administration of oral forms of Ketoconazole;
- hypersensitivity to the drug.
pills are shown for adults and children weighing more than 35 kg.
Lingual pills are shown for adults and children over 5 years.
In children's practice (especially children under 5 years old) It is recommended to use Motilium in the form of a suspension.
In chronic dyspepsia adults and children appoint 10 mg 3 times / day for 15-30 minutes before a meal and, if necessary, before bedtime. The maximum daily dose is 80 mg.
If necessary, for adults and children over the age of 12, the dose can be doubled.
For children the drug in the form of a suspension is prescribed at the rate of 2.5 ml / 10 kg of body weight (which corresponds to 250 μg / kg of body weight) 3 times / day before meals and, if necessary, before going to bed.
If necessary, the indicated dose can be doubled (except for children under 1 year).The maximum daily dose is 2.4 mg / kg body weight, but not more than 80 mg.
With nausea and vomiting adults and children over 12 years old appoint 20 mg 3-4 times / day before meals and at bedtime. The maximum daily dose is 80 mg.
Children aged 5 to 12 years appoint 10 mg 3-4 times / day before meals and at bedtime. The drug in the form of a suspension is prescribed at the rate of 5 ml / 10 kg of body weight (which corresponds to 500 μg / kg of body weight) 3-4 times / day before meals and before bedtime. This dose is achieved by filling the pipette twice. The maximum daily dose is 2.4 mg / kg body weight, but not more than 80 mg.
At renal failure It is recommended to increase the interval between taking the drug. Since Since a very small percentage of the drug is excreted by the kidneys unchanged, there is hardly a need to correct a single dose in patients with renal insufficiency. However, when reappointment frequency should be reduced to 1-2 times / day, depending on the severity of renal failure, may also require dose reduction.
Suspension Application Rules
Shake the vial with suspension before use. The suspension is supplied in a package protected from accidental opening by children, so the bottle should be opened as follows:
1. Press on top of the plastic cap of the bottle, at the same time turning it counterclockwise.
2. Remove the screw cap.
3. Remove the pipette from the case (supplied with a 100 ml bottle).
4. Holding the lower ring in place, lift the upper ring to the mark corresponding to the child's weight (in kg).
5. While holding the bottom ring, pull out the filled pipette from the vial.
6. After use, rinse the pipette with water, place the empty pipette in the pouch and close the bottle.
Rules for the use of lingual tablets
Lingual pills are available in blister packs. Since the instant pills are rather fragile, they should not be pushed through the foil to avoid damage.
In order to get a pill from a blister, you should take the foil by the edge and remove it completely from the cell in which the pill is located. Then gently push the bottom and remove the pill from the package. The pill should be put on the tongue. Within a few seconds, it will disintegrate on the surface of the tongue and it can be swallowed with saliva without drinking water.
Gastrointestinal: rarely - gastrointestinal disorders; in isolated cases - transient spasms of the intestine.
From the side of the central nervous system: extrapyramidal symptoms (very rarely in children; in isolated cases in adults); completely reversible and disappear after cessation of treatment. With insufficient development of the BBB (for example, in children under 1 year of age) or the violation of its functions, the possibility of neurological side effects cannot be completely ruled out.
On the part of the endocrine system: possible hyperprolactinemia, rarely leading to galactorrhea, gynecomastia, amenorrhea.
Allergic reactions: rarely rash, urticaria.
Data on the use of Motilium in pregnancy is not enough.
To date, there is no evidence of increased risk of malformations in humans. However, the use of Motilium in pregnancy (especially in the first trimester) is possible only in cases where the expected benefit of therapy for the mother outweighs the potential risk to the fetus.
In women, the concentration of domperidone in breast milk is 10-50% of the corresponding plasma concentration and does not exceed 10 ng / ml. The total amount of domperidone excreted into breast milk is less than 7 mcg / day when the maximum permissible dose is applied. It is not known whether this level has a negative effect on newborns. Therefore, if it is necessary to use Motilium in the period of lactation, breastfeeding is recommended to be discontinued unless the expected benefit outweighs the potential risk.
In the combined use of Motilium with antacid or antisecretory drugs, the latter should be taken after a meal, i.e. they should not be taken at the same time as Motilium.
With caution should appoint Motilium patients with liver failure, given the high degree of metabolism of domperidone in the liver.
With prolonged therapy, patients should be under regular observation.
Use in pediatrics
Due to the fact that the metabolic processes and function of the BBB in the first months of life are not fully developed, infants should be prescribed any drug very carefully and under careful medical supervision. Sincetypical for Motilium lack of influence on the CNS is mainly the result of poorly pronounced penetration through the BBB, the occurrence of neurological symptoms cannot be completely excluded children under 1 year. Overdose can cause neurological side effects in children.
Influence on ability to drive motor transport and control mechanisms
Motilium does not affect the ability to drive and work with mechanisms.
Symptoms: drowsiness, disorientation and extrapyramidal reactions, especially in children.
Treatment: the use of Activated carbon and careful observation. Anticholinergics, drugs used to treat parkinsonism, or antihistamines can be effective when extrapyramidal reactions occur.
Anticholinergic drugs can neutralize Motilium's anti-dyspeptic effect.
The bioavailability of Motilium when administered orally is reduced after previous administration of cimetidine or sodium bicarbonate. Do not take antacid and antisecretory drugs simultaneously with Motilium, because they reduce its bioavailability.
The main pathway of metabolic transformations of domperidone occurs with the participation of the isoenzyme 3A4 of the cytochrome P system450. On the basis of in vitro studies, it can be assumed that with simultaneous use of domperidone and drugs that significantly inhibit this isoenzyme, plasma domperidone levels may increase.Examples of inhibitors of CYP3A4 isoenzyme are the following drugs: antifungal drugs of the azole series, macrolide antibiotics, HIV protease inhibitors, nefazodone.
When conducting research on healthy volunteers, the interaction of domperidone with ketoconazole revealed that ketoconazole inhibits CYP3A4-dependent primary metabolism of domperidone, which results in an approximately threefold increase in Cmax and AUC of domperidone in the plateau phase. In a study of the interaction of domperidone and ketoconazole, it was shown that when domperidone is used together at a dose of 10 mg 4 times / day and ketoconazole at a dose of 200 mg 2 times / day, the QT interval is prolonged by 10–20 ms. When monotherapy with domperidone in similar doses, and when taking a daily dose of 160 mg (which is 2 times the maximum permissible daily dose), no clinically significant changes in the QT interval were noted.
Theoretically (since the drug has a gastrokinetic effect) Motilium could affect the absorption of concurrently used drugs, in particular, drugs with a slow release of the active substance, or enteric-coated preparations. However, the use of domperidone in patients with Paracetamol or selected therapy with Digoxin did not affect the level of these drugs in the blood.
Motilium can also be combined with neuroleptics, the effect of which it does not enhance; dopaminergic receptor agonists (bromocriptine,levodopa), the undesirable peripheral effects of which, such as digestive disorders, nausea, vomiting, it suppresses without neutralizing their basic properties.
The drug should be stored out of reach of children at a temperature of 15 ° to 30 ° C.
tablets, coated, and suspensions for intake - 5 years, pills lingual - 3 years.
Pharmacy sales terms
Suspension for ingestion is available on prescription.
pills and lingual pills are approved for use as a means of non-prescription.