


 All payments are encrypted via SSL
All payments are encrypted via SSL
 Full Refund if you haven't received your order
Full Refund if you haven't received your order
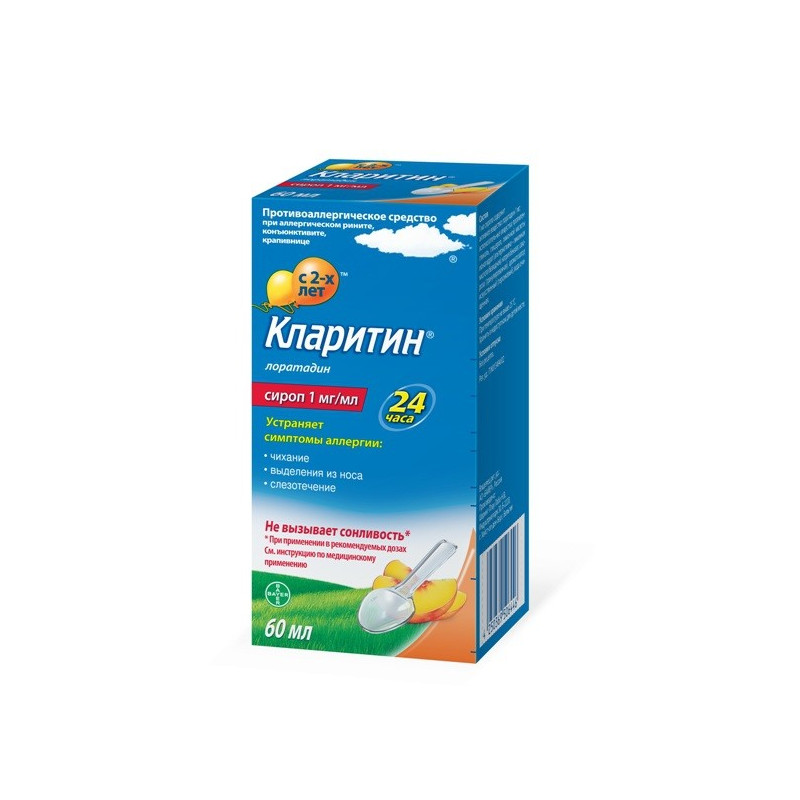
Loratadine.
Syrup.
1 ml of syrup contains
active ingredient: loratadine 1 mg;
excipients: propylene glycol 100 mg, glycerol 100 mg, citric acid monohydrate 9.6 mg (alternatively anhydrous citric acid 8.78 mg), sodium benzoate 1 mg, sucrose (granulated) 600 mg, artificial flavor (peach) 2.5 mg, purified water qs to 1 ml.
Transparent colorless or yellowish syrup that does not contain visible particles.
Antiallergic - H1-histamine receptor blocker
Pharmacodynamics Loratadine, the active substance of the drug Claritin®, is a tricyclic compound with a pronounced antihistamine effect and is a selective blocker of peripheral H1-histamine receptors. It has a fast and long anti-allergic effect. Onset of action - within 30 minutes after taking the drug Claritin® inside. The antihistamine effect reaches a maximum after 8-12 hours from the onset of action and lasts more than 24 hours.
Loratadine does not penetrate the blood-brain barrier and does not affect the central nervous system.It has no clinically significant anticholinergic or sedative effect, i.e. does not cause drowsiness and does not affect the speed of psychomotor reactions when used in the recommended doses. Taking the drug Claritin® does not prolong the QT interval on the ECG.
With long-term treatment, no clinically significant changes in vital signs, physical examination data, laboratory results, or electrocardiography were observed.
Loratadine does not have significant selectivity for H2-histamine receptors. It does not inhibit the reuptake of norepinephrine and has little effect on the cardiovascular system or the function of the pacemaker.
Pharmacokinetics Loratadine is rapidly and well absorbed in the gastrointestinal tract. The time to reach the maximum concentration (Tmax) of loratadine in the blood plasma is 1-1.5 hours, and its active metabolite Desloratadine is 1.5-3.7 hours. Eating increases the time to reach the maximum concentration (Tmax) of loratadine and desloratadine by approximately 1 hour, but does not affect the efficacy of the drug. The maximum concentration (Cmax) of loratadine and desloratadine does not depend on food intake. In patients with chronic kidney disease, the maximum concentration (Cmax) and the area under the concentration-time curve (AUC) of loratadine and its active metabolite are increased compared with patients with normal renal function.The half-lives of loratadine and its active metabolite do not differ from those in healthy patients. In patients with alcoholic lesion of the liver, Cmax and AUC of loratadine and its active metabolite are doubled compared with these indicators in patients with normal liver function.
Loratadine has a high degree (97-99%), and its active metabolite is a moderate degree (73-76%) of binding to plasma proteins.
Loratadine is metabolized to desloratadine through the cytochrome P450 3A4 system and, to a lesser extent, the cytochrome P450 2D6 system. Excreted through the kidneys (approximately 40% of the ingested dose) and through the intestines (approximately 42% of the ingested dose) for more than 10 days, mainly in the form of conjugated metabolites. Approximately 27% of the ingested dose is eliminated through the kidneys within 24 hours after taking the drug. Less than 1% of the active substance is excreted through the kidneys unchanged within 24 hours after taking the drug.
The bioavailability of loratadine and its active metabolite is dose-dependent.
The pharmacokinetic profiles of loratadine and its active metabolite in adult and elderly healthy volunteers were comparable.
The half-life of loratadine is from 3 to 20 hours (an average of 8.4 hours), and desloratadine is from 8.8 to 92 hours (an average of 28 hours); in elderly patients, respectively, from 6.7 to 37 hours (an average of 18.2 hours) and from 11 to 39 hours (an average of 17.5 hours).The half-life increases with alcoholic liver damage (depending on the severity of the disease) and does not change in the presence of chronic renal failure.
Hemodialysis in patients with chronic renal insufficiency does not affect the pharmacokinetics of loratadine and its active metabolite.
Seasonal (pollinosis) and perennial allergic rhinitis and allergic conjunctivitis - elimination of symptoms associated with these diseases - sneezing, itching of the nasal mucosa, rhinorrhea, burning sensation and itching in the eyes, tearing.
Chronic idiopathic urticaria.
Hypersensitivity to loratadine or any other component of the drug.
Children age up to 2 years.
Breastfeeding period.
Patients with deficiency of sucrase / isomaltase, fructose intolerance, glucose-galactose malabsorption - due to the presence of sucrose, which is part of the syrup.
Severe abnormal liver function.
Pregnancy (see section “Use during pregnancy and during breastfeeding”).
The safety of loratadine during pregnancy has not been established. Use of the drug Claritin® in pregnancy is possible only after consulting a doctor if the intended benefit to the mother outweighs the potential risk to the fetus.
Loratadine and its active metabolite are excreted in breast milk, therefore, when prescribing a drug during breastfeeding, the issue of discontinuing breastfeeding should be resolved.
Inside, regardless of meal times.
For adults, including the elderly, and adolescents over the age of 12, it is recommended to take Claritin® at a dose of 10 mg (2 teaspoons (10 ml) of syrup) 1 time per day.
With the use of the drug in elderly patients and in patients with chronic renal failure, dose adjustment is not required.
For children At the age of 2 to 12 years, the dose of Claritin® is recommended to be prescribed depending on body weight:
- 30 kg and less - 5 mg (1 tsp (5 ml) of syrup) 1 time per day;
- more than 30 kg - 10 mg (2 teaspoons (10 ml) of syrup) once a day.
Adults and children with severely impaired liver function, the initial dose should be: with a body weight of 30 kg or less - 5 mg (1 teaspoon (5 ml) of syrup) every other day, with a body weight of more than 30 kg - 10 mg (2 teaspoons ( 10 ml) of syrup) every other day.
In clinical studies involving children aged 2 to 12 years, taking the drug Claritin® more often than in the placebo group (“dummy”), there was a headache (2.7%), nervousness (2.3%), fatigue (one %).
In clinical trials involving adults, adverse events that were observed more frequently than with placebo were seen in 2% of patients taking Claritin®.In adults, when using the drug Claritin® more often than in the placebo group, headache (0.6%), drowsiness (1.2%), increased appetite (0.5%) and insomnia (0.1%) were noted. In addition, in the post-marketing period there were very rare reports (<1/10 000) about dizziness, fatigue, dry mouth, gastrointestinal disorders (nausea, gastritis), allergic reactions in the form of rash, anaphylaxis, including angioedema, alopecia, abnormal liver function, palpitations, tachycardias, and convulsions.
Symptoms: drowsiness, tachycardia, headache.
In case of overdose, you should immediately consult a doctor.
Treatment: symptomatic and supportive therapy. Possible gastric lavage, receiving adsorbents (crushed Activated carbon with water).
Loratadine is not excreted by hemodialysis. After providing emergency care, it is necessary to continue monitoring the patient's condition.
Eating does not affect the effectiveness of Claritin®.
The drug Klaritin® does not strengthen action of alcohol on the central nervous system.
When loratadine was co-administered with Ketoconazole , Erythromycin , or cimetidine, plasma concentrations of loratadine increased, but this increase was not clinically significant, including according to electrocardiography.
Taking Claritin® should be discontinued 48 hours before skin tests, as antihistamine medications may distort the results of a diagnostic study.
No negative effect of the drug Claritin® on the ability to drive a car or perform other activities requiring high concentration of attention was detected.
However, in very rare cases, some patients experience drowsiness while taking Claritin®, which may affect their ability to drive and work with machinery.
Syrup 1 mg / ml. On 60 ml or 120 ml in the bottles of dark glass corked by aluminum screw covers having a ring of the first opening and polyethylene sealing laying, or the polypropylene screw covers having a ring of the first opening, protection against opening of a bottle by children and polyethylene sealing laying. On 1 bottle complete with a plastic spoon batcher or the graduated syringe on 5 ml and the application instruction in a cardboard pack.
At a temperature not higher than 25 ° C. Keep out of the reach of children.
3 years.
Do not use after expiration date.
Over the counter.