

 All payments are encrypted via SSL
All payments are encrypted via SSL
 Full Refund if you haven't received your order
Full Refund if you haven't received your order
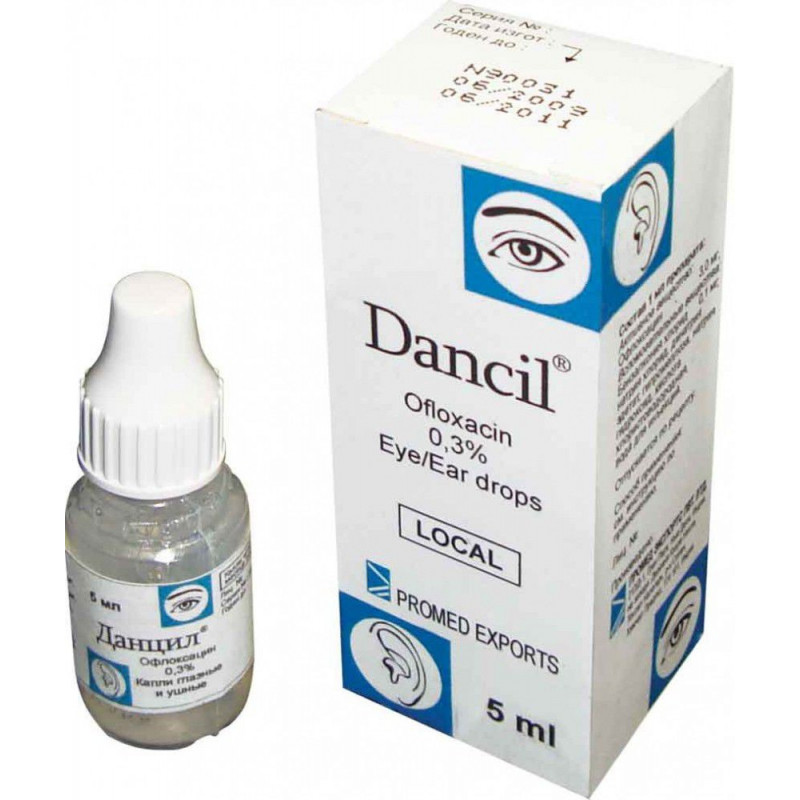
Drops
1 ml of drops contains: ofloxacin-3 mg.
Excipients: benzalkonium chloride, sodium chloride, disodium edetate, hypromellose, sodium hydroxide, hydrochloric acid, water for injection.
In a dropper bottle 5 ml.
DANZIL drops - a broad-spectrum antimicrobial agent from the group of fluoroquinolones, acts on the bacterial enzyme DNA gyrase, which provides supercoiling and so on. DNA stability of bacteria (destabilization of DNA chains leads to their death). It has a bactericidal effect. Sensitive in vivo: Gram-positive aerobes: Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pneumoniae. Gram-negative aerobes: Enterobacter cloacae, Haemophilus influenza, Proteus mirabilis, Pseudomonas aeruginosa, Serratia marcescens. Anaerobes: Propionibacterium acnes. In vitro sensitive: Gram-positive aerobes: Enterococcus faecalis, Staphylococcus hominus, Listeria monocytogenes, Staphylococcus simulans, Staphylococcus capitis, Streptococcus pyogenes. Gram-negative aerobes: Acinetobacter calcoaceticus var. anitratus, Klebsiella pneumoniae, Acinetobacter calcoaceticus var. louffre Other: Chlamydia trachomatis.
Ophthalmology: bacterial corneal ulcers, conjunctivitis, blepharitis, meibomit (barley), dacryocystitis, keratitis, chlamydial eye infections, prevention of infectious complications in the postoperative period after surgical interventions for removing a foreign body and eye injury.ENT-practice: acute and chronic bacterial external and average otitis, otitis with perforation of the eardrum or tympanopuncture; prevention of infectious complications during surgical interventions.
Hypersensitivity (including to other fluoroquinolones, quinolones), epilepsy (including history), impaired function of the central nervous system with a lower threshold of convulsive readiness (including after TBI, stroke, inflammatory processes in the central nervous system), defeat of tendons with previously conducted treatment with fluoroquinolones, age up to 18 years (skeletal growth is not yet completed). For local forms: chronic non-bacterial conjunctivitis or otitis media.
Use during pregnancy is possible (including in the form of topical dosage forms) if the expected effect of therapy outweighs the potential risk to the fetus (adequate and well-controlled studies of the safety of use in pregnant women have not been performed).
Teratogenic effects. Ofloxacin did not have a teratogenic effect when administered to pregnant animals during the period of organogenesis: to rats at doses over 810 mg / kg / day, which is 11 times higher than the Ч rdsh when taken orally and 9000 times when used as eye drops; Rabbits in doses of more than 160 mg / kg / day, which is 4 and 1800 times higher than the MRDC, respectively. Doses equivalent to 50 and 10 mRDF when administered orally were fetotoxic — there was a decrease in fetal body weight and an increase in fetal mortality in rats and rabbits.
Category of action on the fetus by the FDA - C.
With a single dose of 200 mg of ofloxacin by lactating women, its concentrations in breast milk are similar to those in plasma. Since potentially ofloxacin can cause serious adverse reactions in breastfed babies, breastfeeding women should stop either breastfeeding or taking ofloxacin (given the importance of the drug to the mother).
Locally. Floksal: drops are instilled into the conjunctival sac of the affected eye, 1 cap 2-4 times a day for no more than 2 weeks; eye ointment - 1.5 cm strips of ointment are placed 2-3 times a day for the lower eyelid, for chlamydial infections - 5 times a day. The course of treatment is not more than 2 weeks. Uniflox: 1-2 drops to the conjunctival sac every hour for the first 2 days, for the next 2-3 days, 6-7 times a day for 7 days (within 3 days after the cessation of purulent separation). The course of treatment is not more than 2 weeks. In the ENT practice: 10 caps are instilled into the ear canal of the affected ear 2 times a day for 10 days, for chronic purulent otitis media and perforation of the eardrum - for 14 days. A bottle with drops is preheated in the hand for 1-2 minutes (for the prevention of dizziness). The patient must be and remain for 5 minutes after instillation in the "lying on its side".
On the part of the digestive tract: dyspepsia, nausea, vomiting, diarrhea, anorexia, abdominal pain, dry mouth, transient increase in bilirubin and liver enzymes in blood plasma, hepatitis, jaundice, dysbiosis, pseudomembranous colitis.
From the nervous system and sensory organs: dizziness, headache, insomnia, anxiety, slow reaction, agitation, increased intracranial pressure, tremor, convulsions, nightmares, hallucinations, psychosis, paresthesia, phobias, impaired coordination of movements, taste, smell, sight, diplopia, color perception disorders, loss of consciousness.
Since the cardiovascular system and blood (hematopoiesis, hemostasis): cardiovascular collapse, hemolytic and aplastic anemia, thrombocytopenia, including thrombocytopenic purpura, leukopenia, neutropenia, agranulocytosis, pancytopenia.
From the genitourinary system: acute interstitial nephritis, impaired renal excretory function with increased levels of urea and creatinine, vaginitis.
Allergic reactions: skin rash, itching, angioedema, including laryngeal, pharyngeal, face, vocal cords, bronchospasm, urticaria, multiforme exudative erythema, Stevens-Johnson syndrome, toxic epidermal necrolysis, anaphylactic shock.
Other: hypoglycemia (in patients with diabetes mellitus), vasculitis, tendonitis, myalgia, arthralgia, superinfection, photosensitization.
When used in ophthalmology: burning and discomfort in the eyes, redness, itching and dryness of the conjunctiva, photophobia, lacrimation; rarely - dizziness, nausea.
After instillation into the ear canal: itching in the area of the ear canal, feeling of bitter taste in the mouth; rarely, systemic reactions (eczema, dizziness, noise and pain in the ears, dryness of the oral mucosa).
After the disappearance of clinical signs, treatment continues for 2–3 days. With caution prescribed to patients with atherosclerosis of cerebral vessels. Constant monitoring is necessary when combined with insulin, caffeine, theophylline, cyclosporine, NSAIDs, oral anticoagulants (including with warfarin) and drugs metabolized with the participation of cytochrome P450.
In children, it is used only with life threatening (due to the risk of side effects). With rapid i.v. administration, a decrease in blood pressure is possible.
Do not inject subconjunctivally or inject into the anterior chamber of the eye. When using ophthalmic forms, wearing eye lenses is not recommended. Perhaps the combined use of eye drops and eye ointment, while the ointment is used last.
During the period of treatment should not be exposed to solar or UV radiation. It is recommended to refrain from activities requiring quick psychomotor reactions (driving vehicles, working with potentially dangerous mechanisms) and alcohol intake.
After topical application of an excessive dose of Dancil®, the eyes should be washed with clean water at room temperature. There is no data on systemic manifestations of overdose.
In the dark place at a temperature of no higher than 25 ° C (not freezing).
Danzil eye / ear