

 All payments are encrypted via SSL
All payments are encrypted via SSL
 Full Refund if you haven't received your order
Full Refund if you haven't received your order
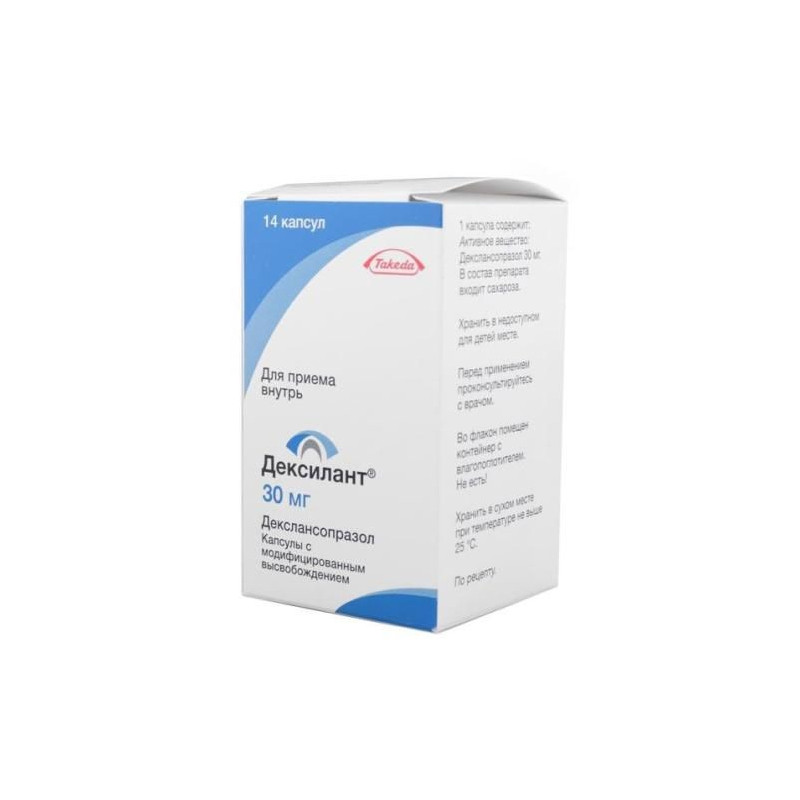
Release form, composition and packaging
Modified release capsules with an opaque blue lid and with an opaque gray body; on the lid in dark gray ink affixed the logo "TAP", on the case - the inscription "30". The contents of the capsules are white to almost white granules.
1 caps | |
dexlansoprazole | 30 mg |
Excipients: Krupkan sugar (from 500 microns to 710 microns) - 28.8 mg (sucrose 18-26.352 mg, corn starch 2.448-10.8 mg), Magnesium carbonate - 11.5 mg, sucrose - 41.5 mg, low-substituted hyprolosis - 8.64 mg, hyprolosis - 0.34 mg, hypromellose 2910 - 7.54 mg, talc - 16.64 mg, titanium dioxide - 5.5 mg, copolymer methacrylic acid dispersion - 9.66 mg (methacrylic acid - 4.4436 mg, ethyl acrylate - 4.2504 mg, sodium lauryl sulfate - 0.2254 mg, polysorbate 80 - 0.7406 mg), macrogol 8000 - 0.96 mg, polysorbate 80 - 0.44 mg, colloidal silicon dioxide - 0.09 mg, methacrylic acid and methyl methacrylate copolymer [1: 2] - 15.95 mg, meth copolymer krilovoy acid and methyl [1: 1] - 5.32 mg of triethyl citrate - 2.12 mg.
The composition of the shell of capsules: carrageenan - 0.192-0.624 mg, potassium chloride - 0.144-0.48 mg, titanium dioxide - 2.4768 mg, FD and C dye blue No. 2 aluminum varnish - 0.3456 mg, iron dye black oxide - 0.0576 mg, hypromellose - q.s. up to 48 mg, peeled gray ink for labeling - trace amounts.
The composition of the ink gray peeled: iron dye oxide red, iron dye oxide yellow, dye FD and C blue No. 2 aluminum varnish, carnauba wax, shellac, glyceryl monooleate.
14 pcs. - polyethylene bottles (1) - packs cardboard.
28 pcs. - polyethylene bottles (1) - packs cardboard.
- treatment of erosive esophagitis of any severity;
- maintenance therapy after treatment of erosive esophagitis and relief of heartburn;
- symptomatic treatment of gastroesophageal reflux disease GERD (ie, NERD - non-erosive reflux disease).
Inside: the capsule is taken entirely regardless of the meal. You can also open the capsule, pour the granules from it into a tablespoon and mix them with applesauce; then swallow immediately, without chewing.
Treatment of erosive esophagitis of any severity: The recommended dose is 60 mg 1 time / day. The course of treatment is 8 weeks.
Maintenance therapy after treatment of erosive esophagitis and relief of heartburn: The recommended dose is 30 mg 1 time / day. In the studies, the course of treatment was up to 6 months.
At moderate and severe erosive esophagitis The recommended dose is 60 mg 1 time / day. In the studies, the course of treatment was up to 6 months.
Symptomatic treatment of gastroesophageal reflux disease GERD (ie, NERD - non-erosive reflux disease): pThe recommended dose is 30 mg 1 time / day. The course of treatment is 4 weeks.
In patients with impaired liver function of moderate severity (class B on Child-Pugh) daily dose should not exceed 30 mg of dexlansoprazole.
Clinical data on taking the drug in patients with severe disorders (class C on Child-Pugh) are absent.
Dose adjustment in elderly patients patients with impaired renal function and with impaired liver function severity (class A on Child-Pugh) is not required.
Most often (at least 2%): diarrhea, flatulence, abdominal pain, nausea, vomiting, upper respiratory tract infections.
Determination of the frequency of adverse reactions: very often (≥1 / 10), often (≥1 / 100 and <1/10), infrequently (≥1 / 1000 and <1/100), rarely (≥1 / 10 000 and <1 / 1000), very rarely (<1/10 000, including individual cases), the frequency is unknown (it is impossible to estimate based on available data).
On the part of the immune system: frequency is unknown - hypersensitivity (including anaphylactic reactions), malignant exudative erythema (Stevens-Johnson syndrome), toxic epidermal necrolysis, exfoliative dermatitis, anaphylactic shock.
Metabolism: frequency is unknown - hypomagnesemia, hyponatremia.
From the digestive system: often - diarrhea, discomfort and pain in the abdomen, constipation, flatulence, nausea, vomiting; infrequently - dry mouth; rarely - oral candidiasis; frequency is unknown - swelling of the oral mucosa, pancreatitis.
From the urinary system: frequency unknown - acute renal failure.
Liver and biliary tract: infrequently - changes in the indicators of the functional activity of the liver; frequency unknown - drug hepatitis.
Skin and Subcutaneous Tissues: infrequently - a rash, hives, itching; frequency is unknown - leukocytoclastic vasculitis, generalized rash.
On the part of the respiratory system: often - infectious diseases of the upper respiratory tract; infrequently - cough; frequency is unknown - swelling of the larynx, feeling of tightness in the throat.
From the blood and lymphatic system: frequency unknown - autoimmune hemolytic anemia, idiopathic thrombocytopenic purpura.
From the musculoskeletal system: frequency unknown - fractures.
Since the cardiovascular system: infrequently - attack of heat ("hot flashes"), increased blood pressure.
From the nervous system: often - headache; infrequently - dizziness, dysgeusia; rarely - paresthesia, convulsions; frequency unknown - stroke, transient ischemic attack.
On the part of the organ of vision: rarely - visual impairment; frequency unknown - blurred vision (misting).
On the part of the organ of hearing and labyrinth disorders: rarely - vertigo; frequency is unknown - decrease in hearing.
Mental Disorders: infrequently - insomnia, depression; rarely auditory hallucinations.
Common disorders: infrequently - weakness, changes in appetite; frequency unknown - swelling of the face.
- Hypersensitivity to any of the components of the drug;
- combined use with HIV protease inhibitors (atazanavir, nelfinavir);
- age up to 18 years;
- pregnancy;
- lactation period.
The drug contains sucrose, so its use is not recommended for patients with hereditary fructose intolerance, glucose-galactose malabsorption or sugar-isomalt insufficiency.
Carefully:
- patients taking tacrolimus;
- patients taking CYP2C19 isoenzyme inhibitors, such as fluvoxamine;
- patients taking Warfarin (under the control of prothrombin time and MHO);
- patients taking Methotrexate.
Use of the drug Dexilant ® during pregnancy is contraindicated. If necessary, the use of the drug during lactation should stop breastfeeding.
In patients with impaired liver function of moderate severity (class B but Child-Pugh), the daily dose should not exceed 30 mg of dexlansoprazole.
Clinical data on the drug in patients with severe disorders (class C on Child-Pugh) are not available.
Dose adjustment in patients with mild hepatic impairment (Child-Pugh class A) is not required.
Dose adjustment in patients with impaired renal function is not required.
Contraindicated in children under 18 years.
Dose adjustment in elderly patients is not required.
Before starting treatment with dexlansoprazole, the possibility of a malignant neoplasm should be excluded, since the drug can mask the symptoms and delay the correct diagnosis.
If symptoms persist despite adequate treatment, further examination should be carried out.
When taking proton pump inhibitors, which include dexlansoprazole, increases the risk of gastrointestinal infections, accompanied by diarrhea, the causative agents of which are bacteria of the genus Clostridium difficile, especially in hospitalized patients. This should be taken into account if the patient’s condition does not improve in the treatment of diarrhea.
Patients in this case are advised to take the minimum effective dose of dexlansoprazole with the shortest duration of treatment.
In patients receiving high doses of the drug or with prolonged therapy with proton pump inhibitors (PPI) for a year or more, the risk of osteoporotic fractures of the thighs, hands and spine increases. Patients at risk for osteoporotic fractures should adhere to the recommended doses.
In rare cases, patients experienced symptomatic and asymptomatic hypomagnesemia when taking drugs IIT for at least 3 months, and in most cases - when taking for a year. Symptoms of hypomagnesemia are tetany, arrhythmia, and seizures. Treatment is the replenishment of magnesium and the discontinuation of taking drugs IDU. In patients who need long-term treatment or at the same time taking drugs IIT with Digoxin or other drugs that can cause hypomagnesemia (for example, diuretics), it is necessary to control the concentration of magnesium in the serum before and during treatment.
Influence on ability to drive motor transport and control mechanisms
Because of the likelihood of dizziness and visual impairment, patients during the treatment period should refrain from driving and other mechanisms requiring increased attention.
Overdose
Reports of significant cases of overdose as a result of the use of the drug Dexilant® not noted.
Symptoms: repeated doses of 120 mg and a single dose of 300 mg did not cause serious side effects. When taking the drug Dexilant® 60 mg 2 times / day was observed increase in blood pressure more than 140/90 mm Hg.
Treatment: in case of overdose and only in the presence of clinical manifestations, symptomatic therapy is carried out. Dexansoprazole is not excreted by hemodialysis.
Drug interaction
Dexansoprazole can be administered without the risk of drug interaction to patients taking Clopidogrel. In the case of co-administration, clopidogrel dose adjustment is not required.
The absence of clinically significant drug interactions with phenytoin, theophylline and diazepam was also noted.
The simultaneous use of dexlansoprazole may affect the absorption of drugs whose bioavailability depends on the pH of the stomach (for example, ampicillin esters, digoxin, iron salts, Ketoconazole, erlotinib).
Simultaneous administration with tacrolimus can lead to an increase in plasma tacrolimus concentration, especially in post-transplant patients who are moderate or slower metabolizers of the CYP2C19 isoenzyme.
When taken concurrently with fluvoxamine, there is a possibility of an increase in systemic exposure to dexlansoprazole.
Simultaneous administration of dexlansoprazole and methotrexate can lead to an increase and maintenance of a high concentration of methotrexate and / or its metabolite in serum. If you need to receive high doses of methotrexate, it is recommended to temporarily discontinue dexlansoprazole.
Pharmacy sales terms
The drug is available on prescription.
Terms and conditions of storage
The drug should be kept out of the reach of children, in a dry place at a temperature not exceeding 25 ° C.