


 All payments are encrypted via SSL
All payments are encrypted via SSL
 Full Refund if you haven't received your order
Full Refund if you haven't received your order
Hyalgan Phidia is an intra-articular injection containing sodium hyaluronate solution. The solution is injected by the doctor directly into the sore joint and provides recovery of the intra-articular fluid, thereby helping to cope with pain and impaired joint mobility in osteoarthritis or after injuries.
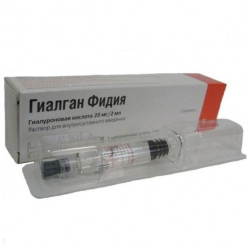
In our pharmacy, the drug Gialgan Phidia is sold in a form convenient for use - a filled syringe. The needle for the introduction of the drug is chosen by the doctor individually, depending on the size of the joint and the patient's complexion.
1 ml of solution contains sodium hyaluronate 10 mg.
Colorless, transparent, viscous solution of 2 ml in a syringe without a needle.
Hyaluronic acid is a necessary component of the extracellular matrix, is present in high concentrations in the composition of the articular cartilage and synovial fluid. Endogenous hyaluronic acid provides the viscosity and elasticity of synovial fluid, and it is also necessary for the formation of proteoglycans in articular cartilage.
In osteoarthritis, there is a deficiency and qualitative changes in hyaluronic acid in the composition of the synovial fluid and cartilage.Intra-articular injection of hyaluronic acid on the background of degenerative changes in the surface of the synovial cartilage and the pathology of synovial fluid leads to an improvement in the functional state of the joint.
With the use of Hyalgan Phidia, an improvement in the clinical course of osteoarthrosis has been observed for 6 months from the moment of treatment, an anti-inflammatory and analgesic effect has been observed.
The drug is intended for intraarticular injection.
In the knee and hip joints, the drug is administered in a dose of 20 mg 1 time per week (contents of 1 filled syringe). The course consists of 3-5 injections.
Rules of drug administration
It is necessary to follow the rules of asepsis and antiseptics when performing the procedure.
Before the introduction of the hyalgan phidium, remove the effusion from the articular sac. Introduce the drug should be exactly in the joint cavity by standard methods, taking into account the anatomical features. To remove the effusion and administer the drug, you can use the same needle, which was inserted once before aspiration. In this case, the syringe with the drug should be attached to the needle, released from the syringe with aspirated fluid. To confirm the location of the needle in the joint cavity, it is necessary to aspirate the available amount of synovial fluid before the drug is slowly injected. The introduction of the drug into the joint cavity should be discontinued when pain occurs during the injection.
Avoid getting air into the syringe with the drug.
Remaining in the syringe, the drug can not be stored.
Use during pregnancy and lactation: do not prescribe the drug during pregnancy and lactation (breastfeeding) due to the lack of clinical data on the use of this category of patients.
During the first 2 days after the procedure, it is recommended not to overload the joint, especially to avoid prolonged activity.
Upon receipt of the aspiration fluid before the introduction of the drug should conduct appropriate studies to exclude bacterial etiology of arthritis.
Use in pediatrics: due to the lack of clinical data on the use in pediatrics, the drug should not be prescribed to children.
Influence on the ability to drive motor vehicles and control mechanisms: Gialgan Phidia does not affect the ability to drive motor vehicles and to other potentially dangerous activities that require increased concentration of attention and psychomotor speed.
The drug should be stored out of the reach of children, protected from light at a temperature not exceeding 25 ° C; do not freeze.Shelf life - 3 years when stored in its original packaging.
Do not use the drug Gialgan Phidia with damaged or opened package.
Disinfectants containing quaternary ammonium salts should not be used, since hyaluronic acid precipitates in the presence of these substances.
