


 All payments are encrypted via SSL
All payments are encrypted via SSL
 Full Refund if you haven't received your order
Full Refund if you haven't received your order
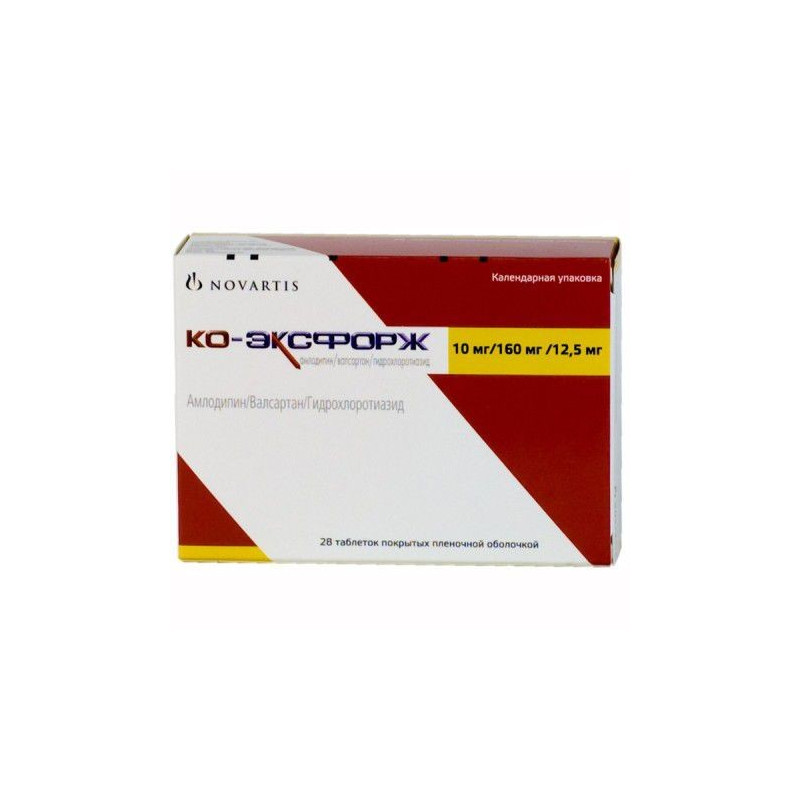
Release form, composition and packaging
Film Coated Tablets white, oblong, biconvex, with beveled edges, embossed with "NVR" on one side and "VCL" on the other.
Excipients: microcrystalline cellulose, crospovidone, colloidal silicon dioxide, Magnesium stearate.
The composition of the film shell: hypromellose, titanium dioxide (E171), macrogol, talc, iron dye yellow oxide (E172), iron dye red oxide (E172).
pharmachologic effect
Co-Exforge is a combination of three antihypertensive components with a BP control mechanism that complements each other: Amlodipine (a derivative of dihydropyridine) - a blocker of slow Calcium channels, valsartan - angiotensin II receptor antagonist (ATII) and hydrochlorothiazide- thiazide diuretic. The combination of these components leads to a more pronounced decrease in blood pressure compared with that on the background of monotherapy with each drug separately.
Amlodipine, which is part of Co-Exforge, inhibits transmembrane entry of calcium ions into cardiomyocytes and vascular smooth muscle cells. The mechanism of antihypertensive action of amlodipine is associated with a direct relaxing effect on vascular smooth muscle, causing a decrease in round neck and a decrease in blood pressure.
After administration in therapeutic doses in patients with arterial hypertension, amlodipine causes dilation of blood vessels, leading to a decrease in blood pressure (in the patient's position "lying" and "standing"). Lowering blood pressure is not accompanied by a significant change in heart rate and catecholamine activity with prolonged use.
Plasma concentrations of the drug correlate with the therapeutic response in both young and elderly patients.
In hypertensive patients with normal renal function, amlodipine at therapeutic doses leads to a decrease in renal vascular resistance, an increase in glomerular filtration rate and effective plasma renal blood flow without changing the filtration fraction and the severity of proteinuria.
Also, as with the use of other slow calcium channel blockers, amlodipine in patients with normal left ventricular function was observed to change the hemodynamic parameters of heart function at rest and during exercise: a slight increase in cardiac index, without significant effect on the maximum rate of pressure rise in left ventricle, on the end-diastolic pressure and volume of the left ventricle. Hemodynamic studies in intact animals and healthy volunteers showed that a decrease in blood pressure under the influence of amlodipine in the range of therapeutic doses is not accompanied by a negative inotropic effect, even when used simultaneously with beta-blockers.
Amlodipine does not change the function of the sinoatrial node and does not affect the AV conductivity in intact animals and healthy volunteers. When using amlodipine in combination with beta-blockers in patients with arterial hypertension or with angina, the decrease in blood pressure is not accompanied by undesirable changes in electrocardiographic parameters.
The clinical efficacy of amlodipine in patients with stable angina, vasospastic angina, and angiographically confirmed coronary artery disease has been proven.
In a prolonged placebo-controlled study (PRAISE-2) in patients with chronic heart failure (III and IV functional class according to the NYHA classification) of non-ischemic etiology, with the use of amlodipine, there was an increase in the incidence of pulmonary edema, in the absence of significant differences heart failure compared with placebo.
The risk of myocardial infarction or an increase in the severity of angina: rarely at the beginning of therapy with slow calcium channel blockers or with an increase in their dose (especially in patients with hypertrophic obstructive cardiomyopathy, severe obstructive coronary artery disease), there was an increase in the frequency, duration and severity of angina attacks or acute myocardial infarction. Arrhythmia (including ventricular tachycardia and atrial fibrillation) has also been noted with the use of slow calcium channel blockers. These adverse events were impossible to differentiate from the natural course of the disease.
Valsartan is an active and specific angiotensin II receptor antagonist, intended for oral administration. It acts selectively on AT subtype receptors.1which are responsible for the effects of angiotensin II. Increased plasma concentration of unbound angiotensin II due to blockade of AT1receptors influenced by valsartan can stimulate unlocked AT2receptors that counteract the effects of AT stimulation1-receptors. Valsartan does not have any pronounced agonistic activity against AT1-receptors. The affinity of valsartan to receptors of the AT subtype1 about 20,000 times higher than the AT subtype receptors2.
Valsartan does not inhibit ACE, which converts angiotensin I to angiotensin II and causes the destruction of bradykinin. Since When using angiotensin II antagonists, ACE inhibition and accumulation of bradykinin or substance P do not occur, the development of dry cough is unlikely.
In comparative clinical studies of valsartan with an ACE inhibitor, the incidence of dry cough was significantly lower (p <0.05) in patients who received valsartan (in 2.6% of patients who received valsartan and in 7.9% who received an ACE inhibitor). In a clinical study that included patients who had previously developed a dry cough when treated with an ACE inhibitor, this complication was observed in 19.5% of cases in treatment with valsartan, and in 19.0% of cases in the treatment with thiazide diuretic.At the same time, in the group of patients who received treatment with an ACE inhibitor, cough was observed in 68.5% of cases (p <0.05). Valsartan does not interact and does not block the receptors of other hormones or ion channels that are important for regulating the functions of the cardiovascular system.
When treating valsartan in patients with arterial hypertension, a decrease in blood pressure is observed, which is not accompanied by a change in heart rate.
The antihypertensive effect appears within 2 hours in most patients after a single dose of valsartan inside. The maximum decrease in blood pressure develops after 4-6 hours. After taking valsartan, the duration of the hypotensive effect lasts for more than 24 hours. With repeated use, the maximum reduction in blood pressure, regardless of the dose, is usually reached within 2-4 weeks and maintained at the reached level during long-term therapy. Abrupt cessation of valsartan is not accompanied by a sharp increase in blood pressure or other undesirable clinical consequences. The use of valsartan in patients with chronic heart failure (functional class II-IV according to the NYHA classification) leads to a significant reduction in the number of hospitalizations for cardiovascular diseases (which is especially pronounced in patients not receiving ACE inhibitors or beta-adrenergic blockers). When taking valsartan in patients with left ventricular failure (stable clinical course) or with impaired left ventricular function after myocardial infarction, a decrease in cardiovascular mortality is noted.
The point of application of the action of thiazide diuretics are distal convoluted renal tubules. When thiazide diuretics are exposed to highly sensitive receptors of the distal tubules of the cortical layer of the kidneys, the reabsorption of sodium ions (Na+) and chlorine (Cl-). Suppression of the co-transport system Na+ and Сl-, apparently, occurs due to competition for Cl binding sites- in this system. As a result, the excretion of sodium ions and chlorine increases approximately equally. As a result of the diuretic effect, a decrease in BCC is observed, as a result of which renin activity increases, aldosterone secretion, kidney excretion of potassium, and, consequently, a decrease in the content of potassium in blood serum.
The use of triple combination therapy with amlodipine + valsartan + hydrochlorothiazide showed a more pronounced decrease in systolic and diastolic blood pressure, compared with the use of double combinations: valsartan + hydrochlorothiazide, amlodipine + valsartan and amlodipine + hydrochlorothiazide. In patients with grade II and III arterial hypertension (baseline mean blood pressure of 170/107 mm Hg) when using combination therapy with amlodipine + valsartan + hydrochlorothiazide in a daily dose of 10 mg + 320 mg + 25 mg for 8 weeks, the average decrease in systolic and diastolic blood pressure was 39.7 / 24.7 mm Hg. (compared with 32.0 / 19.7 mm Hg, 33.5 / 21.5 mm Hg., 31.5 / 19.5 mm Hg.against the background of combination therapy, valsartan + hydrochlorothiazide at a dose of 320 mg + 25 mg, amlodipine + valsartan at a dose of 10 mg + 320 mg and amlodipine + hydrochlorothiazide at a dose of 10 mg + 25 mg, respectively).
The greatest antihypertensive effect of Co-Exforge is observed 2 weeks after the start of use of the drug in the maximum individual oral dose.
When using Co-Exforge, the achievement of target blood pressure (less than 140/90 mm Hg) was noted in 71% of patients, compared with 45-54% against the background of the use of double combinations.
After taking the drug, the antihypertensive effect lasts for 24 hours.
The therapeutic efficacy of Co-Exforge does not depend on the age, gender and race of patients.
Pharmacokinetics
Pharmacokinetic parameters of amlodipine, valsartan and hydrochlorothiazide are characterized by linearity.
Suction
After oral administration of amlodipine in therapeutic doses Cmax in blood plasma is achieved in 6-12 hours. Absolute bioavailability is on average 64-80%. Eating does not affect the bioavailability of amlodipine.
Distribution
Vd is approximately 21 l / kg. In vitro studies of amlodipine have shown that in patients with arterial hypertension, approximately 97.5% of the circulating drug binds to plasma proteins .
Metabolism
Amlodipine is extensively (approximately 90%) metabolized in the liver to form active metabolites.
Removal
Withdrawal from plasma is biphasic with T1/2 approximately 30 to 50 hours. Css in blood plasma are achieved after prolonged use for 7-8 days.10% is displayed in unchanged form, 60% - in the form of metabolites.
Suction
After ingestion of valsartan Cmax in blood plasma is achieved in 2-4 hours. The average absolute bioavailability is 23%.
The pharmacokinetic curve of valsartan is of a descending multiexponential nature (T1/2α <1 h and T1/2β about 9 hours). When taken with food, there is a decrease in bioavailability (by AUC value) by 40% and Cmax in the blood plasma by almost 50%, although approximately 8 hours after taking the drug inside the concentration of valsartan in the blood plasma of people who took it with food, and in the group who received the drug on an empty stomach, are aligned. AUC reduction, however, is not accompanied by a clinically significant decrease in the therapeutic effect, therefore, valsartan can be prescribed regardless of the meal time.
Distribution
Vd Valsartan in the equilibrium state after the on / in the introduction was about 17 liters, which indicates the absence of an extensive distribution of valsartan in the tissues. Valsartan is largely associated with serum proteins (94-97%), mainly with albumin.
Metabolism
Valsartan does not undergo a pronounced metabolism (about 20% of the dose taken is determined as metabolites). The hydroxyl metabolite is detected in plasma in low concentrations (less than 10% of the AUC of valsartan). This metabolite is pharmacologically inactive.
Removal
Valsartan is excreted mainly unchanged through the intestines with feces (about 83% of the dose) and with the kidneys (about 13% of the dose).After intravenous injection, plasma clearance of valsartan is about 2 l / h and its renal clearance is 0.62 l / h (about 30% of the total clearance). T1/2 is 6 hours
Suction
Hydrochlorothiazide absorption after oral administration is fast (time to reach Cmax about 2 hours). On average, the increase in AUC is linear and proportional to the dose in the therapeutic range. With simultaneous ingestion of food, both an increase and a decrease in the systemic bioavailability of hydrochlorothiazide were reported as compared with fasting. The magnitude of this effect is small and clinically insignificant. The absolute bioavailability of hydrochlorothiazide after oral administration is 60-80%.
Distribution
The kinetics of distribution and elimination is generally described as a biexponential decreasing function, with T1/2 6-15 hours. With repeated use, the kinetics of hydrochlorothiazide does not change and when used once a day, the accumulation is minimal. Seeming vd - 4-8 l / kg. 40-70% of the hydrochlorothiazide circulating in the blood plasma is bound to plasma proteins, mainly albumin. Hydrochlorothiazide also accumulates in erythrocytes in concentrations of approximately 3 times higher than in plasma.
Metabolism
Hydrochlorothiazide is excreted unchanged.
Removal
More than 95% of the absorbed dose of hydrochlorothiazide is excreted unchanged by the kidneys in the urine.
After ingestion of the drug Co-Exforge Cmax amlodipine, valsartan and hydrochlorothiazide are reached after 6-8, 3 and 2 h, respectively. The speed and degree of absorption of Co-Exforge are equivalent to the bioavailability of amlodipine, valsartan and hydrochlorothiazide when taking each of them in separate tablets.
Pharmacokinetics in special clinical situations
Pharmacokinetic features of the use of Co-Exforge in children under 18 years have not been established.
Time to reach Cmax amlodipine in the blood plasma of young and elderly patients alike. In elderly patients, the clearance of amlodipine is slightly reduced, which leads to an increase in AUC and T1/2. In elderly patients, systemic exposure to valsartan was slightly more pronounced than in younger patients, however, this was not clinically significant. There is limited evidence of a decrease in the systemic clearance of hydrochlorothiazide in patients over 65 years of age (healthy or with arterial hypertension) compared with younger patients.
In patients with impaired renal function, the pharmacokinetic parameters of amlodipine do not significantly change. There was no correlation between renal function (CK) and systemic exposure of valsartan (AUC) in patients with varying degrees of renal dysfunction. However, since the excretion of hydrochlorothiazide occurs mainly through the kidneys, impaired renal function can have a significant effect on the pharmacokinetics of hydrochlorothiazide.
Patients with impaired liver function have reduced clearance of amlodipine, which leads to an increase in AUC of approximately 40-60%. On average, in patients with liver disorders with mild (5-6 points on the Child-Pugh scale) and moderate (7-9 points on the Child-Pugh scale), the bioavailability (by AUC) of valsartan is doubled compared with healthy volunteers (appropriate age , sex and body weight).
Dosage
The drug should be taken orally (preferably in the morning), washed down with a small amount of water, regardless of the meal.
For convenience, patients receiving therapy with amlodipine, valsartan and hydrochlorothiazide in separate tablets can be transferred to therapy with Co-Exforge, containing the same doses of active ingredients, as well as in case of insufficient blood pressure control against the background of double combined therapy (valsartan + hydrochlorothiazide, amlodipine + valsartan and amlodipine + hydrochlorothiazide), patients can be transferred to a triple combination treatment with Co-Exforge in appropriate doses.
If a patient has dose-dependent side effects when using dual-combination therapy with any of the components of Co-Exforge, Co-Exforge may be prescribed to achieve a similar reduction in blood pressure, containing a lower dose of the active component that caused this side effect.
The recommended daily doses of Co-Exforge are:
- 5 mg + 160 mg + 12.5 mg (1 tab. containing amlodipine + valsartan + hydrochlorothiazide in doses of 5 mg + 160 mg + 12.5 mg);
- 10 mg + 160 mg + 12.5 mg (1 tab. containing amlodipine + valsartan + hydrochlorothiazide in doses of 10 mg + 160 mg + 12.5 mg);
- 10 mg + 320 mg + 25 mg (2 tab. containing amlodipine + valsartan + hydrochlorothiazide in doses of 5 mg + 160 mg + 12.5 mg).
The maximum antihypertensive effect of the drug is observed 2 weeks after increasing the dose. The maximum dose of the drug is 10 mg + 320 mg + 25 mg / day.
Have patients over 65 dose adjustment of the drug is not required.
Since the safety and efficacy of Co-Exforge children and adolescents (under 18) not yet established, the drug is not recommended for use in this category of patients.
Have sick with mild and moderate renal dysfunction (CC more than 30 ml / min) and liver (5-9 points on the Child-Pugh scale) dose adjustment of the drug is not required.
Overdose
Data on cases of overdose of the drug are currently not available.
With an overdose valsartan can be expected to develop a pronounced decrease in blood pressure and dizziness.
Overdose amlodipine may result in excessive peripheral vasodilation and possible reflex tachycardia. It was also reported about the occurrence of a pronounced and prolonged decrease in blood pressure up to the development of a fatal shock.
The main clinical manifestations of overdose hydrochlorothiazide are symptoms associated with loss of electrolytes (hypokalemia, hypochloremia) and dehydration due to stimulation of diuresis. The most common symptoms of an overdose are nausea and drowsiness.Hypokalemia may be accompanied by muscle spasms. With the concomitant use of cardiac glycosides (or other antiarrhythmic drugs), hypokalemia can increase cardiac arrhythmia.
Treatment: in case of accidental overdose, induce vomiting (if the drug has been taken recently) or gastric lavage. The use of Activated carbon in healthy volunteers immediately or 2 hours after taking amlodipine significantly reduced its absorption. With a marked decrease in the patient's blood pressure, the patient should be laid with raised legs, and active measures should be taken to increase blood pressure and maintain the cardiovascular system, including regular monitoring of heart and respiratory system function, BCC and the amount of urine released. In the absence of contraindications to restore vascular tone and blood pressure, it is possible to use (with caution) a vasoconstrictor. Intravenous administration of calcium salt solutions can be effective in eliminating calcium channel blockade. Removal of valsartan and amlodipine during hemodialysis is unlikely. Hydrochlorothiazide can be removed from the systemic circulation by hemodialysis.
Drug interaction
Amlodipine
When monotherapy with amlodipine is not observed clinically significant interaction with thiazide diuretics, beta-blockers, ACE inhibitors, long-acting nitrates, Nitroglycerin for sublingual use, Digoxin , Warfarin , Atorvastatin , Sildenafil ,antiacid preparations (magnesium hydroxide, aluminum hydroxide gel, simethicone), cimetidine, NSAIDs , antibiotics and hypoglycemic preparations for oral administration.
Inhibitors of the isoenzyme CYP3A4 . When using amlodipine together with diltiazem, in elderly patients, amlodipine metabolism slows down, probably due to inhibition of CYP3A4 isoenzyme, which leads to an increase in plasma concentration of amlodipine by approximately 50% and an increase in its systemic exposure. When using amlodipine together with powerful inhibitors of CYP3A4 (for example, Ketoconazole , itraconazole and ritonavir), a marked increase in systemic exposure to amlodipine is possible.
Inductors of isopermept CYP3A4. Since the use of amlodipine together with inducers of CYP3A4 isoenzyme (for example, Carbamazepine , phenobarbital, phenytoin, phosphenytoin, primidone, rifampicin, grapefruit juice, herbal preparations containing St. John's wort), can cause a marked decrease in blood plasma, containing St. John's wort hollowed out), can cause a dosage of application in a blood plasma that is perforated, hovering holed), with dosage levels in the blood plasma, caused by the use of dosage in the form of a dosage, holed, hovering holed) with a dosage CYP3A4, its plasma levels should be monitored.
Valsartan
It was established that in monotherapy with valsartan there is no clinically significant interaction with the following drugs: cimetidine, warfarin, Furosemide , digoxin, Atenolol , Indomethacin , hydrochlorothiazide, amlodipine, glibenclamide.
When applied simultaneously with dietary supplements containing potassium,Care should be taken to regularly monitor capillary levels in the blood, with potassium-sparing diuretics, potassium-containing salt substitutes, or with other drugs that may cause an increase in the level of potassium in the blood (for example, with heparin).
When using valsartan with NSAIDs, it is possible to reduce the antihypertensive effect of valsartan.
Hydrochlorothiazide
Lithium. With simultaneous use with ACE inhibitors and diuretics, cases of a reversible increase in the plasma concentration of lithium and its toxic action have been reported. Therefore, with simultaneous use of hydrochlorothiazide and lithium preparations, it is recommended to control the concentration of lithium in the blood plasma.
Peripheral muscle relaxants. Thiazide diuretics, including hydrochlorothiazide, potentiate the action of peripheral muscle relaxants.
NSAIDs. It is possible to reduce the diuretic and antihypertensive effects of the thiazide component of Co-Exforge when used simultaneously with NSAIDs, for example, with Acetylsalicylic acid , indomethacin. Concomitant hypovolemia can lead to the development of acute renal failure .
Drugs that can cause an increase in plasma potassium. The risk of hypokalemia increases with the simultaneous appointment of other diuretics, GCS, ACTH, amphotericin B, carbenoxolone and acetylsalicylic acid (at a dose of more than 3 g).Caution should be exercised with simultaneous use of Co-Exforge with potassium salts, potassium-sparing diuretics, potassium-containing substitutes for edible salt, as well as drugs that can cause an increase in blood potassium (for example, heparin).
Cardiac glycosides. Thiazide diuretics can cause undesirable effects such as hypokalemia or hypomagnesemia; These conditions increase the risk of developing arrhythmias with simultaneous use of cardiac glycosides.
Hypoglycemic agents for oral administration and insulin . When using the drug in patients with diabetes mellitus, it may be necessary to adjust the dose of insulin or hypoglycemic agents for oral administration. Since the use of hydrochlorothiazide together with Metformin may develop lactic acidosis (due to dysfunction during hydrochlorothiazide therapy), caution should be exercised when using the drug Co-Exforge in patients receiving treatment with metformin.
Anticholinergics. It is possible to increase the bioavailability of thiazide diuretic with simultaneous use of m-anticholinergics (for example, atropine, biperiden), which, apparently, is associated with a decrease in gastrointestinal motility and a slower rate of gastric emptying.
Methyldopa Cases of hemolytic anemia have been reported with simultaneous administration of hydrochlorothiazide and methyldopa.
Kolestiramine reduces the absorption of thiazide diuretics, including hydrochlorothiazide.
Vitamin D and calcium salts. The combined use of thiazide diuretics, including hydrochlorothiazide, with vitamin D or calcium salts may increase the calcium content in blood serum.
Cyclosporine. Simultaneous administration of cyclosporine may increase the risk of developing hyperuricemia and the appearance of symptoms resembling the worsening of gout.
Carbamazepine. In patients taking hydrochlorothiazide concurrently with carbamazepine may develop hyponatremia. Since patients who are receiving hydrochlorothiazide and carbamazepine at the same time may develop hyponatremia, appropriate administration of plasma sodium should be carried out when prescribing Co-Exforge along with carbamazepine.
Other types of interaction. The simultaneous appointment of thiazide diuretics, including hydrochlorothiazide, may lead to an increase in the frequency of hypersensitivity reactions to Allopurinol ; increased risk of side effects of amantadine; increased hyperglycemic action of diazoxide; reducing kidney excretion of cytotoxic agents (eg, cyclophosphamide, methotrexate) and potentiating their myelosuppressive action.
Pregnancy and lactation
It is known that the appointment of ACE inhibitors that affect the RAAS, pregnant in the II and III trimesters, leads to damage or death of the developing fetus.Given the mechanism of action of angiotensin II receptor antagonists, the risk to the fetus cannot be excluded. According to a retrospective analysis of the use of ACE inhibitors in the first trimester of pregnancy was accompanied by the development of pathology of the fetus and newborn. Hydrochlorothiazide penetrates the placental barrier. When using thiazide diuretics, including hydrochlorothiazide, pregnancy may develop embryonic or neonatal thrombocytopenia, as well as other undesirable reactions observed in adult patients. In case of unintentional use of valsartan in pregnant women, cases of spontaneous abortions, low water, and impaired renal function in newborns are described. Co-Exforge, like any other drug that has a direct effect on the RAAS, should not be prescribed during pregnancy and women planning pregnancy.
should be informed about the possible risk to the fetus associated with the use of drugs that affect the RAAS. If pregnancy is diagnosed during the period of treatment with Co-Exforge, the drug should be canceled as soon as possible.
It is not known whether valsartan and / or amlodipine passes into breast milk. In experimental studies, the release of valsartan with breast milk was noted. Hydrochlorothiazide is excreted in breast milk. Co-Exforge should not be used during breastfeeding.
Side effects
Below are all adverse events that were observed with simultaneous use of amlodipine, valsartan and hydrochlorothiazide (Co-Exforge), as well as monotherapy with amlodipine, valsartan and hydrochlorothiazide.
Co-Exforge (amlodipine + valsartan + hydrochlorothiazide)
The safety of Co-Exforge was evaluated in more than 2,200 patients. When using the drug Co-Exforge, adverse events were mostly few or moderately severe. Termination of drug treatment due to the development of adverse events was required in rare cases. Most often, the drug was discontinued due to the development of dizziness and a pronounced decrease in blood pressure (0.7%).
When using the drug Co-Exforge, no new adverse events were detected as compared with dual combination therapy and monotherapy with individual components.
As with the short-term intake, the good tolerability of Co-Exforge was observed with its long-term use (for a year).
The incidence of adverse events was not related to gender, age or race.
With the use of Co-Exforge, changes in laboratory parameters were minimal and did not differ from those on the background of monotherapy with individual components. While taking hydrochlorothiazide together with valsartan (triple combination therapy), a decrease in the hypokalemic effect of hydrochlorothiazide is noted.
The most frequent adverse events (frequency more than 2%), noted in clinical studies (regardless of the identification of a connection with Co-Exforge), were dizziness (7.7%), peripheral edema (4.5%), headache (4.3%), dyspepsia (2.2%), fatigue (2.2%), muscle spasm (2.2%), back pain (2.1%), nasopharyngitis (2.1%), nausea (2.1%).
The following criteria were used to estimate the frequency (according to the WHO classification): very often (≥1 / 10); often (≥1 / 100, <1/10); infrequently (≥1 / 1000, <1/100); rarely (≥1 / 10,000, <1/1000); very rarely (<1/10 000), frequency unknown (not enough data to estimate the frequency of development).
Metabolism: often - hypokalemia; infrequently - anorexia, hypercalcemia, hyperlipidemia, hyponatremia.
From the nervous system: often - dizziness, headache; infrequently - insomnia / sleep disorders, lack of coordination, postural dizziness and dizziness due to exercise, taste disorders, lethargy, paresthesias, neuropathy, incl. peripheral, drowsiness, fainting.
From the senses: infrequently - visual disturbances, vertigo.
Since the cardiovascular system: often - a pronounced decrease in blood pressure; infrequently - tachycardia, orthostatic hypotension, phlebitis, thrombophlebitis.
On the part of the respiratory system: infrequently - cough, shortness of breath, irritation in the throat.
From the digestive system: often - dyspepsia; infrequently - abdominal discomfort, pain in the upper abdomen, bad breath, diarrhea, dry mouth, nausea, vomiting.
Dermatological reactions: infrequently - increased sweating, itching.