

 All payments are encrypted via SSL
All payments are encrypted via SSL
 Full Refund if you haven't received your order
Full Refund if you haven't received your order
medicinal product for medical use
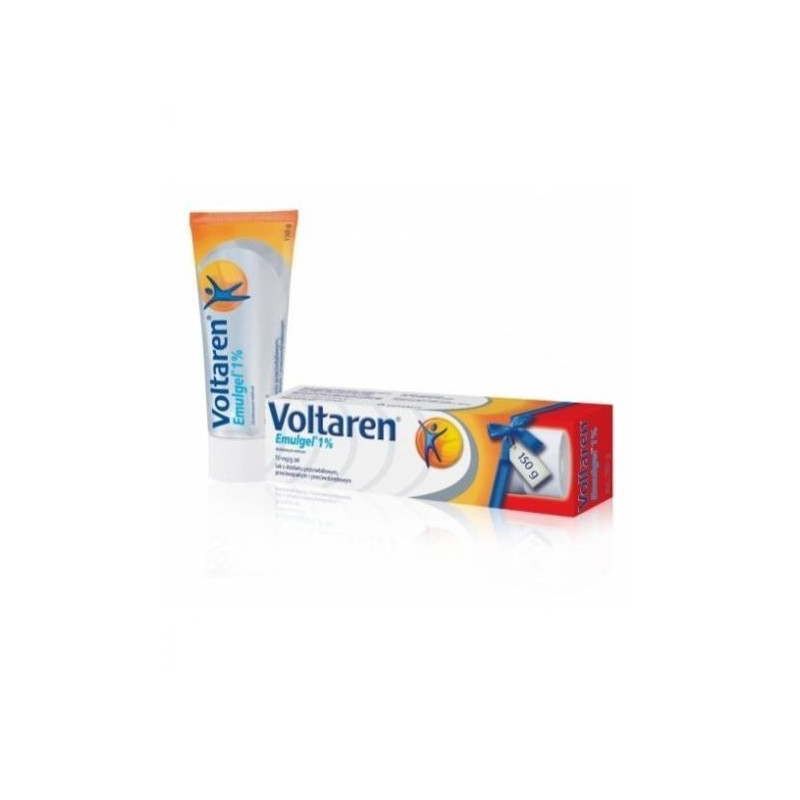
VOLTAREN EMULGEL
Diclofenac
Chemical name: 2 - [(2, 6-dichlorophenyl) amino] benzeneacetic acid (in the form of sodium salt)
Dosage form: gel for external use
100 g of the preparation contain:
Active substance: 1.16 g of Diclofenac diethylamine, which corresponds to 1 g of diclofenac sodium.
Excipients: carbomers (carbopol 974 P) 1.20 g, macrogol cetostearate (cetomacrogol 1000) 2.00 g, cocoyl caprylocaprate (cetiol LC) 2.50 g, diethylamine 0.90 g, isopropanol 20.00 g, liquid paraffin 2.50 g, aromatic cream 45 (contains benzyl benzoate) 0.10 g, propylene glycol 5.00 g, water 64.64 g
Uniform, creamy gel, color from white to yellowish.
Nonsteroidal anti-inflammatory drug (NSAIDs).
Pharmacodynamics
The active ingredient diclofenac is a non-steroidal anti-inflammatory drug that has pronounced analgesic and anti-inflammatory properties. Indirectly inhibiting cyclo-oxygenase 1 and type 2, violates the metabolism of arachidonic acid. Voltaren® Emulgel® used to eliminate pain and reduce swelling associated with the inflammatory process.
Pharmacokinetics
With the recommended method of application of the drug is absorbed no more than 6% diclofenac.When applied to the area of the affected joint, the concentration of synovial fluid is higher than in plasma.
Diclofenac is predominantly distributed and retained deep in tissues susceptible to inflammation, such as joints, where its concentration is 20 times higher than in plasma.
Hypersensitivity to diclofenac or other components of the drug; a tendency to the occurrence of asthma attacks, skin rashes or acute rhinitis with the use of Acetylsalicylic acid or other NSAIDs; pregnancy (III trimester), breastfeeding; children's age (up to 12 years); violation of the integrity of the skin in the intended place of application.
Hepatic porphyria (exacerbation), erosive and ulcerative lesions of the gastrointestinal tract, severe impaired liver and kidney function, chronic heart failure, bronchial asthma, old age, pregnancy (I and II trimester).
Due to the lack of data on the use of Voltaren® Emulgel® in pregnant women, the use of the drug during the I and II trimester of pregnancy is recommended only on prescription, comparing the benefits to the mother and the risk to the fetus.
The drug is contraindicated in the third trimester of pregnancy due to the possibility of lowering the tone of the uterus and / or premature closure of the arterial duct of the fetus.
Due to the lack of data on the penetration of Voltaren® Emulgel® in breast milk, the drug is not recommended for use during breastfeeding. If you still need to use the drug, then it should not be applied on the mammary glands or on a large surface of the skin and not to apply for a long time.
Outwardly.
For adults and children over 12 years old, the drug is applied to the skin 3-4 times a day and lightly rubbed. The required amount of the drug depends on the size of the painful area. A single dose of the drug - 2-4 g (which is comparable in volume, respectively, with the size of a cherry or walnut). Wash your hands after applying the product. The duration of treatment depends on the indications and the effect noted (to enhance the effect, the gel can be used together with other Voltaren dosage forms®). Consult a doctor after 2 weeks in the absence of a therapeutic effect.
Laminated tubes: to remove the protective membrane, use a screw cap as a key (recess with protrusions on the outside of the cap).Align the groove on the outside of the lid with the shaped protective membrane of the tube and turn. The membrane should separate from the tube.
Laminated tubes can have either a regular cover (round shape) or an innovative cover (triangular shape), which is especially suitable for use with limited mobility of the joints of the hands due to osteoarthritis or other joint diseases or injuries.
Aluminum Tubes: Before first use, the protective membrane of the tube should be pierced with a special protrusion on the outside of the polypropylene screw cap.
Undesirable reactions are mainly characterized by moderately pronounced and passing skin manifestations at the site of application of the gel.
Frequent manifestations (≥1/100, < 1/ 10):
Local reactions: eczema, dermatitis, incl. contact dermatitis (symptoms: erythema, pruritus, swelling of the treated skin area, rash, papules, vesicles, desquamation).
Rare manifestations (≥1/10000, 1/1000):
Local reactions: bullous dermatitis.
Very rare manifestations (<1/10000):
Local reactions: pustular rash.
Systemic reactions: generalized skin rash; allergic reactions (kiv-pivnitsa, hypersensitivity: angio-neurotic edema, asthma attacks, bronchospastic reactions), photosensitization reactions.
Extremely low systemic absorption of the active components of the drug for external use makes overdosing almost impossible.However, with the occasional use of 100 g of gel equivalent to 1000 mg of diclofenac, undesirable reactions similar to side reactions may occur.
First aid is to wash the stomach, taking Activated carbon. If necessary, inpatient treatment.
Voltaren® Emulgel® may enhance the effect of drugs that cause photosensitivity. Clinically significant interactions with other drugs are not described.
Voltaren® Emulgel® should be applied only to intact skin, avoiding contact
on open wounds. After application, do not apply an occlusive dressing. Do not allow the drug to enter the eyes and mucous membranes. The drug contains propi-glycol and Benzyl benzoate, which in some cases can cause mild local skin irritation.
Does not affect.
Gel for external use of 1%.
10 g, 20 g, 50 g or 100 g in an aluminum tube equipped with a protective membrane made of aluminum, with a screw-on plastic cap (white or blue), with a protrusion for perforation of the membrane from the outside. The tube together with the instructions for use is placed in a cardboard box.
10 g, 20 g, 50 g or 100 g in a laminated tube (low density polyethylene, aluminum, high density polyethylene) with a shoulder and solid molded protective membrane made of high density polyethylene and a polypropylene screw cap (white or blue), round or triangular shape.The lid on the outside is provided with a key (recess with protrusions) for opening the protective membrane of the tube. The tube together with the instructions for use is placed in a cardboard box.
75 ml or 100 ml in an aluminum bottle (about 73 g and 97 g, respectively) under pressure with a plastic pump dispenser on a plastic ring and a protective cap. The bottle together with the application instruction is placed in a cardboard pack.
At a temperature not higher than 30 ° С, out of the reach of children.
3 years. The drug should not be used after the expiration date indicated on the package.
Over the counter.
