

 All payments are encrypted via SSL
All payments are encrypted via SSL
 Full Refund if you haven't received your order
Full Refund if you haven't received your order
capsules
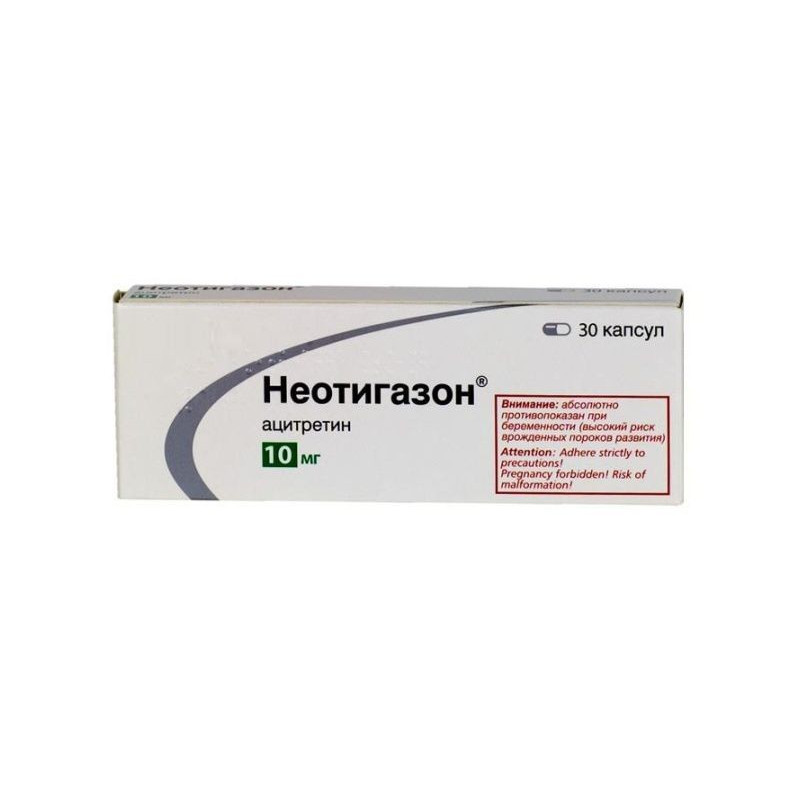
One capsule of Neotigason contains: acitretin 10 mg.
Excipients: microcrystalline cellulose, maltodextrin, gelatin, sodium ascorbate.
In the package of 30 capsules.
Neotigason - systemic retinoid.
Atsitretin, the active ingredient of the drug Neotigason, is a synthetic aromatic analogue of retinoic acid. In preclinical studies on the tolerability of the drug Neotigason, no mutagenic or carcinogenic effect was found; there was also no indication of its direct hepatotoxicity. Neotigason had a pronounced teratogenic effect on animals.
Clinical studies have confirmed that in case of psoriasis and disturbances of keratinization, the drug Neotigason normalizes the processes of proliferation, differentiation and keratinization of the cells of the epidermis, and its side effects are, in general, quite tolerable. The action of the drug is purely symptomatic; its mechanism remains largely unknown.
Severe forms of psoriasis, including psoriatic erythroderma, localized or generalized pustular psoriasis.
Severe dyskeratosis, such as congenital ichthyosis; red hair lichen; Daria's disease; other severe violations of keratinization, resistant to traditional therapies.
Hypersensitivity to the drug (Neotigason or fillers) or to other retinoids; severe hepatic and renal failure; severe chronic hyperlipidemia.
Neotigason has a strong teratogenic effect and should not be given to pregnant women. The same applies to all women capable of childbearing, unless they use reliable contraceptives four weeks before the start of treatment with Neotigason, during treatment and for two years after its completion.
Neotigason is highly teratogenic.It is contraindicated not only to pregnant women and women who may become pregnant during treatment or within 2 years after its termination, but also to all women potentially capable of having children. The risk of having a child with developmental disabilities is especially high if Neotigason is taken before or during pregnancy, regardless of dose and duration of therapy. The effect of Neotigason on the fetus is always associated with the risk of congenital malformations.
Neotigason contraindicated to any woman capable of childbearing, unless each of the following conditions are met:
The patient suffers a severe violation of keratinization, resistant to standard types of treatment.
You can be sure that the patient understands and follows the instructions of the doctor.
The patient is able to carefully and continuously apply the prescribed contraceptives.
It is absolutely necessary that every woman capable of childbirth use effective contraceptives without interruption for 4 weeks before the start of treatment, during the treatment and for two years after the completion of the treatment with Neotigason.
Neotigason treatment should not begin before the 2nd or 3rd day of the next normal.
menstrual cycle.
Two weeks before the start of treatment with Neotigason, a negative pregnancy test should be obtained. During treatment, it is recommended to conduct additional tests for pregnancy at least 1 time per month.
Before starting treatment with Neotigason, the doctor should give detailed information, verbally and in writing, to women capable of childbearing about the necessary precautions, the dangers of very severe fetal malformations and the possible consequences of pregnancy during Neotigason treatment or within 2 years after it ends.
The same effective and continuous contraceptive measures should be applied every time the course of treatment with Neotigason is repeated, regardless of its duration, and should be observed for two years after the end of the course.
If, despite all precautions, during pregnancy with Neotigason or during 2 years after its termination, pregnancy occurs, there is a great risk of severe fetal malformations (for example, a hernia of the brain).
Neotigason should not be prescribed to nursing mothers.
Women of childbearing age should not drink alcohol during the treatment with Neotigason, since there is clinical evidence that etretinate may form in the body if you take Neotigason and alcohol. The mechanism of this metabolic transformation is not established; therefore, it is unclear whether other substances can participate in it. Reception of ethanol should be avoided for 2 months after discontinuation of therapy with Neotigason.
Women of childbearing age can not be blood transfused from patients receiving the drug Neotigason. Therefore, during the treatment with Neotigason and during the year after its completion, blood donation is prohibited.
Liver function should be monitored before starting treatment with Neotigason, every 1-2 weeks during the first month after starting treatment, and then every 3 months. If test results indicate pathology, monitoring should be carried out weekly. If liver function does not return to normal or deteriorates further, Neotigason should be withdrawn. In this case, it is recommended to continue to monitor liver function for at least 3 more months.
It is necessary to control the level of cholesterol and fasting serum triglycerides, especially in patients at risk (lipid metabolism disorders, diabetes, obesity, alcoholism) and with long-term treatment with Neotigason.
In patients with diabetes mellitus, retinoids can improve or worsen glucose tolerance, therefore, in the early stages of treatment with Neotigason, the glucose concentration in the blood should be checked more often than usual.
Adults receiving long-term therapy with Neotigason should regularly conduct appropriate examinations, taking into account the possibility of ossification abnormalities (see “Side Effects”). In the event of such violations should be discussed with the patient the question of continuing treatment, carefully matching the possible risks and benefits of the drug.
In children, you need to carefully monitor the parameters of growth and bone development.
Because of the possibility of impaired night vision, patients should be warned about the need for caution when driving a car or working with machines and mechanisms at night.Care should be taken to monitor visual impairment.
Currently, not all the effects of the use of the drug Neotigason, which may occur throughout life, are known.
In the event of an acute overdose, the drug Neotigone should be discontinued immediately. No other special measures are required, since the acute toxicity of the drug is low. Overdose symptoms are identical to those in acute hypervitaminosis A (headache, dizziness).
Store Neotigason at a temperature not exceeding 25 ° C, in a place protected from light and moisture. Keep out of the reach of children.
