

 All payments are encrypted via SSL
All payments are encrypted via SSL
 Full Refund if you haven't received your order
Full Refund if you haven't received your order
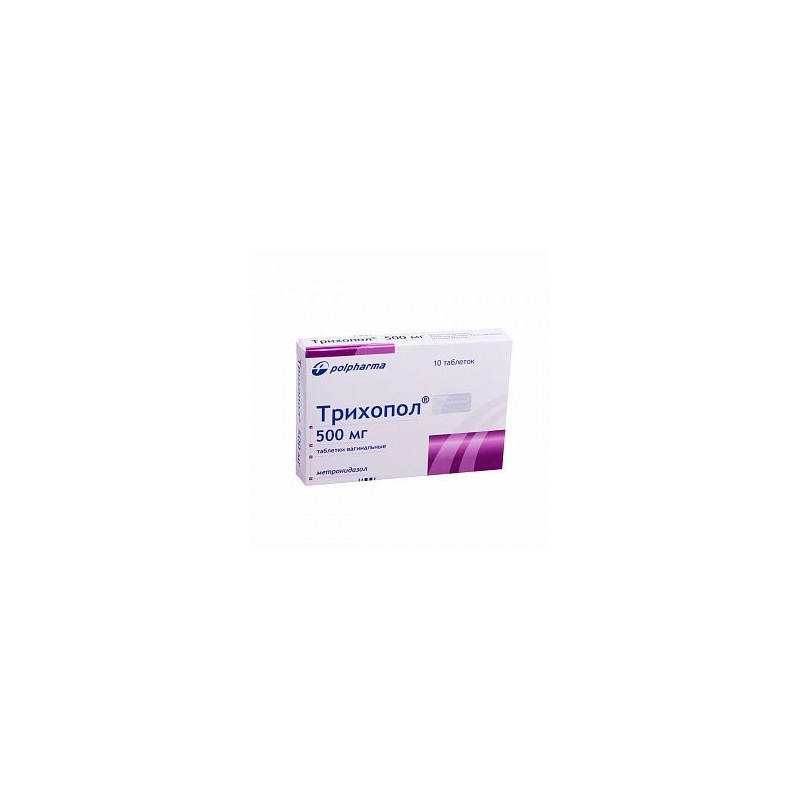
Trichopol vaginal tablets white color with a yellowish shade, oblong, with rounded ends.
1 pill contains Metronidazole 500 mg;
auxiliary substances: rice starch, gelatin, starch syrup, Magnesium stearate.
10 pieces. in a blister, 1 blister in a carton box.
Trichopol is an antimicrobial and antiprotozoal drug of a wide spectrum of action, a derivative of 5-nitroimidazole.
The mechanism of action of metronidazole is the biochemical reduction of the 5-nitro group of metronidazole by intracellular transport proteins of anaerobic microorganisms and protozoa. The restored 5-nitro group of metronidazole interacts with the microbial cell DNA, inhibiting the synthesis of their nucleic acids, which leads to the death of microorganisms.
Active against Trichomonas vaginalis, Giardia intestinalis, Entamoeba histolytica, Lamblia intestinalis, and in relation to obligate anaerobes (spore-and non-spore-forming) - Bacteroides spp. (Bacteroides fragilis, Bacteroides distasonis, Bacteroides ovatus, Bacteroides thetaiotaomicron, Bacteroides vulgatus), Fusobacterium spp., Clostridium spp., Peptostreptococcus spp., Eubacterium spp. Sensitive strains.
To metronidazole are resistant aerobic microorganisms and facultative anaerobes.
For the topical treatment of non-specific vaginitis; Trichomonas vaginitis.
Prevention of anaerobic infections during surgical interventions on the abdominal organs and small pelvis.
Contraindications
- blood diseases;
- leukopenia (including in history);
- incoordination;
- organic lesions of the central nervous system (including epilepsy);
- hepatic impairment (for administration in high doses);
- I trimester of pregnancy;
- lactation (breastfeeding);
- hypersensitivity to metronidazole or other nitroimidazole derivatives.
With care - renal / hepatic failure.
Dosage and administration
When nonspecific vaginitis appoint 1 pill of vaginal in combination with 2 tab. Trichopolum for oral administration per day for 7 days or 1 pill of vaginal 2 times / day.
The duration of treatment is not more than 10 days, treatment should be carried out no more than 2-3 times a year.
With trichomonas vaginitis appoint 1 pill of vaginal per day for 10 days in combination with taking metronidazole inside.
The vaginal pill should be removed from the contour packaging, moistened with boiled chilled water and inserted deep into the vagina.
Gastrointestinal: nausea, taste changes, metallic taste in the mouth, dry mouth, loss of appetite, cramped abdominal pain, nausea, vomiting, constipation or diarrhea.
Nervous system: headache, irritability, sleep disturbances, dizziness, ataxia, depression, peripheral neuropathy, convulsions, tinnitus, hearing loss, disorientation, fainting.
Hemic and lymphatic: transient leukopenia and thrombocytopenia; described the case of bone marrow aplasia.
Allergic reactions: skin rash, itching, urticaria.
Local reactions: itching, burning, pain and irritation in the vagina; thick, white, mucous discharge from the vagina (odorless or with a faint odor), frequent urination; after discontinuation of the drug - the development of vaginal candidiasis.
Other: rarely, urine staining in red-brown color due to the presence of a water-soluble pigment resulting from the metabolism of metronidazole; burning sensation or irritation of the penis of the sexual partner.
Patients with a history of indications of side effects from the central nervous system and hematopoietic system during treatment with metronidazole, if necessary, re-use should be under close medical supervision.
When prescribing Trichopol, patients with impaired liver function should correct the dosage regimen of the drug due to the possible cumulation of metronidazole in the body.
Precautions should be prescribed the drug to patients prone to the appearance of edema or patients receiving GCS because of the high sodium content in the solution.
In the period of use of Trichopol there is a darker staining of urine.
On the background of drug therapy, it is possible to obtain false results in determining the activity of liver transaminases, LDH, triglyceride levels and glucose in the blood plasma.
Metronidazole can immobilize treponemas, which leads to a false-positive TPI test (Nelson's test).
It is necessary to avoid simultaneous use of Trikhopol and indirect anticoagulants. If necessary, their joint appointment should be carefully monitored prothrombin time and set the appropriate dose of anticoagulant.
Caution should be used Trichopol simultaneously with lithium preparations, while the need to control the concentration of lithium and creatinine in the blood plasma.
Do not use Trichopol simultaneously with astemizole and terfenadine.
Trichopolum can be used no earlier than 2 weeks after the end of the disulfiram intake.
In combination with Amoxicillin is not recommended to use the drug in patients younger than 18 years.
In case of leukopenia, the possibility of continuing treatment depends on the risk of developing an infectious process.
After giardiasis treatment, if symptoms persist, after 3–4 weeks to perform 3 fecal tests at intervals of several days (some successfully treated patients may have lactose intolerance caused by invasion, which may persist for several weeks or months, recalling the symptoms of giardiasis) a history of changes in the composition of peripheral blood, as well as with the use of the drug in high doses and / or with long-term treatment, control of the complete blood count is necessary.
During the period of treatment with Trichopolum, alcohol should be avoided, since Acetaldehyde accumulation may occur due to impaired ethanol oxidation. As a result, the development of antabus-like reactions is possible.
In the treatment of Trichomonas vaginitis in women and Trichomonas urethritis in men must refrain from sexual activity. Necessarily simultaneous treatment of sexual partners.
The treatment does not stop during menstruation.
After treatment of trichomoniasis, control tests should be carried out during three regular cycles before and after menstruation.
Sulfonamides enhance the antimicrobial action of metronidazole.
With simultaneous use Trichopolum potentiates the action of Warfarin and other indirect anticoagulants and increases the prothrombin time.
With the simultaneous use of disulfiram and Trichopol may develop acute psychosis, disorientation.
With short-term use of Trichopol on the background of treatment with lithium salts in high doses, it is possible to increase the concentration of lithium in the blood plasma, increase its toxicity and the appearance of symptoms of renal dysfunction.
With the simultaneous use of Trichopol with astemizole and terfenadine, ECG changes, arrhythmias, heart block, syncope are possible.
Pharmacokinetic interaction
With the simultaneous use of Trichopol with cimetidine, the metabolism of metronidazole in the liver can be reduced, which can lead to a delayed removal and an increase in the concentration of metronidazole in blood plasma.
With the simultaneous use of Trichopol and phenobarbital, the metabolism of metronidazole is accelerated due to the induction of microsomal liver enzymes, resulting in T1/2 and plasma plasma concentrations of metronidazole decrease.
With the simultaneous use of Trichopol and phenytoin, a decrease in clearance and an increase in plasma concentration of phenytoin is observed.
The drug should be stored in a dry, dark place at temperatures not above 25 ° C.
